Your Platform to Shape the Future of Predictive Toxicology & NAM Adoption
As biopharma faces unprecedented pressure to improve clinical safety prediction, reduce late-stage attrition, and respond to rapidly evolving regulatory expectations, toxicology leaders are actively reassessing who they partner with and why. Pharma and biotech teams are no longer looking for one-off services; they are seeking trusted, scientifically rigorous partners who can help them interpret risk, validate innovation, and make confident IND-enabling decisions across in vivo,in vitro, and in silico toxicology.
This meeting is the only industry-led forum bringing together senior toxicologists, translational safety leaders, and regulatory influencers at the exact moment the FDA and global agencies are accelerating the shift toward NAMs, advanced in vitro systems, and AI-enabled models. By partnering, you gain direct access to the scientists shaping internal decision-making frameworks, model qualification strategies, and vendor selection across big pharma and high growth biotechs.
Align your brand with real scientific challenges, real data, and real regulatory conversations and position your organization as a long-term strategic partner, not just a service provider.

What to Expect?
Meet New Clients
Direct access to decision-makers across investigative, translational, and regulatory toxicology from leading pharma and biotech actively evaluating new vendors and platforms
Centre Yourself in Industry Dealmaking
High-value visibility across all three solution provider types: in vivo CROs, in vitro/NAM developers, and computational & in silico solution providers
Demonstrate Your Expertise
A platform to showcase scientific credibility, model performance, and real-world application—not marketing hype—to an audience demanding validation and transparency
Expect High Levels of Engagement
Meaningful engagement around model qualification, regulatory relevance, and fit-for-purpose use, aligned with FDA, IQ MPS, and VICT3R perspectives
Shape Future Partnerships
Opportunities to shape future partnerships and pilot collaborations as attendees look to de-risk programs, fill model gaps, and build integrated safety strategies
Key Interest Areas
1
In Vivo Toxicology & Translational CROs – GLP and non-GLP in vivo models across small and large species, supporting dose selection, target safety, biodistribution, and IND-enabling toxicology with regulatory-relevant endpoints and proven execution under tight timelines.
2
In Vitro & NAM Platforms – Advanced human-relevant assays, organoids, and microphysiological systems that enable early hazard identification, mechanism-based risk assessment, and smarter candidate prioritization aligned with the 3Rs principles and emerging regulatory expectations.
3
Computational & In Silico Toxicology Solutions – Predictive modeling, AI-enabled analytics, and virtual control strategies that integrate historical in vivo data, chemical structure, and in vitro outputs to improve decision-making, reduce uncertainty, and support next-generation safety strategies.
Hear What Our Past Sponsors Have to Say

First attendance and I learned a lot with the extremely instructive and focused presentations. Also, the organization allowed to have many opportunities to meet each others for business and partnering
XenTech (From Hanson Wade’s 13th Tumor Models Boston Summit 2025)
Audience Composition
Company Type
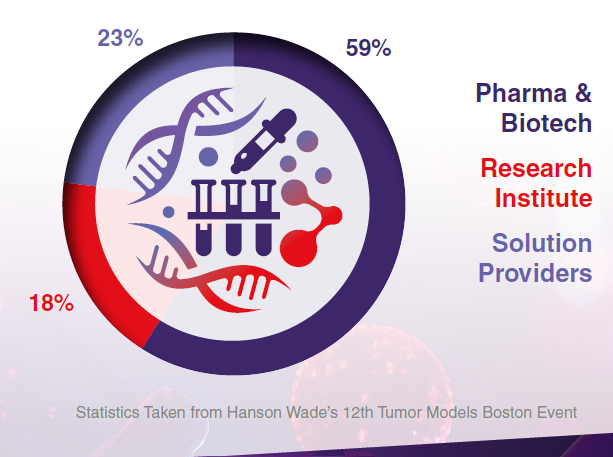
Attendee Seniority
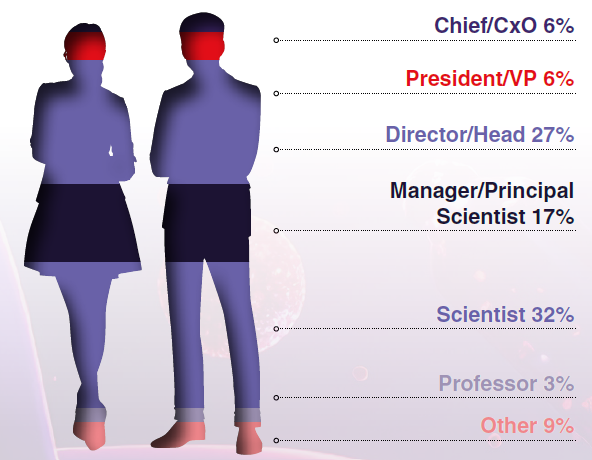
(Graphics From Hanson Wade’s 13th Tumor Models Boston Summit 2025)
Attending Companies Include









Available Inventory
We offer a flexible range of sponsorship opportunities designed to help you demonstrate scientific leadership, engage decision-makers, and align your brand with the future of toxicology innovation - from high-visibility packages to bespoke activations.







Whether your goal is lead generation, thought leadership, or long-term strategic partnerships, we’ll work with you to build a package that delivers measurable impact.
Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.

Marinela Sealey
Business Development Manager
